September 2025
Aktuelles aus Nordamerika
In den USA werden bei der Identifizierung von Gefahren in Verbraucherprodukten diese zurückgerufen und im Consumer Product Safety Commission (CPSC) Recent Recalls auf der CPSC-Website veröffentlicht, die täglich aktualisiert wird. Die US-Rückrufe vom 01. August 2025 bis 31. August 2025 sind unten zusammengefasst:
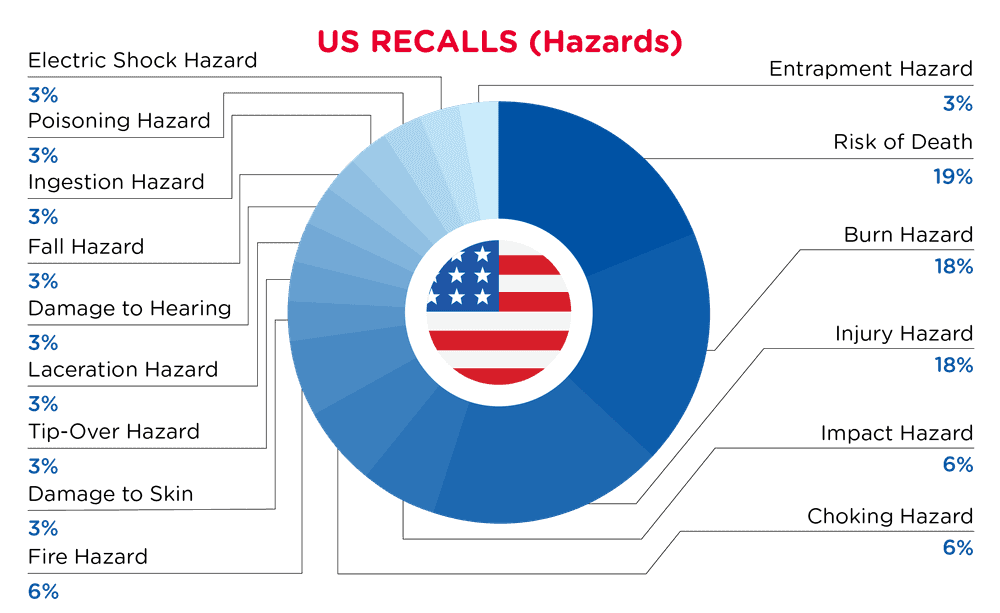
| Gefahren | Frequenz |
| Risiko des Todes | 6 |
| Verbrennungsgefahr | 6 |
| Verletzungsgefahr | 6 |
| Auswirkung Gefährdung | 2 |
| Erstickungsgefahr | 2 |
| Brandgefahr | 2 |
| Schädigung der Haut | 1 |
| Gefahr des Umkippens | 1 |
| Gefahr von Schnittverletzungen | 1 |
| Schädigung des Gehörs | 1 |
| Sturzgefahr | 1 |
| Gefahr des Verschluckens | 1 |
| Gefahr von Vergiftungen | 1 |
| Gefahr eines elektrischen Schlages | 1 |
| Gefahr des Einklemmens | 1 |
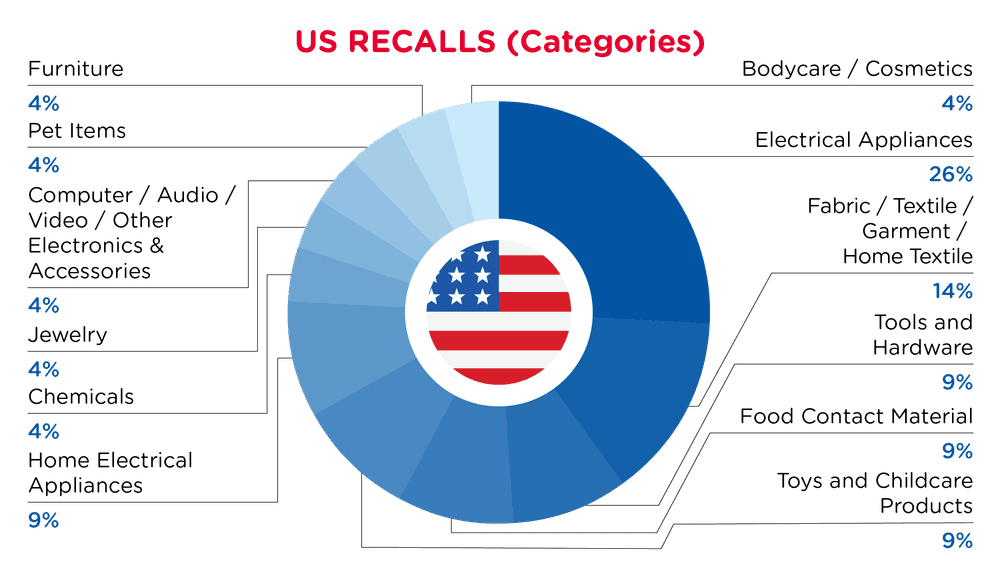
| Produkt-Kategorien | Frequenz |
| Elektrische Geräte | 5 |
| Stoff / Textil / Bekleidung / Heimtextilien | 3 |
| Werkzeuge und Hardware | 2 |
| Material mit Lebensmittelkontakt | 2 |
| Spielzeug und Kinderpflegeprodukte | 2 |
| Elektrische Haushaltsgeräte | 2 |
| Chemikalien | 1 |
| Schmuck | 1 |
| Computer / Audio / Video / Sonstige Elektronik & Zubehör | 1 |
| Artikel für Haustiere | 1 |
| Möbel | 1 |
| Körperpflege / Kosmetika | 1 |
Für eine vollständige Liste klicken Sie hier
Der Bundesstaat Washington hat eine neue Regel eingeführt, die das absichtliche Hinzufügen von Formaldehyd und bestimmten formaldehydfreisetzenden Chemikalien (bekannt als Formaldehydfreisetzungen) in kosmetischen Produkten verbietet. Diese Maßnahme soll die öffentliche Gesundheit schützen, indem sie die Exposition gegenüber diesen potenziell krebserregenden Stoffen reduziert. Die Verordnung betrifft Produkte, die im Bundesstaat verkauft, hergestellt oder vertrieben werden, einschließlich derjenigen, die in professionellen Dienstleistungen, im Online-Verkauf und in Einzelhandelsgeschäften verwendet werden.
Formaldehyd ist ein farbloses, stark riechendes chemisches Mittel, das historisch in vielen Branchen als Konservierungs- und Desinfektionsmittel verwendet wurde. In Kosmetika wurde es häufig direkt oder indirekt (durch formaldehydfreisetzende Konservierungsmittel) hinzugefügt, um mikrobielles Wachstum zu verhindern und die Haltbarkeit zu verlängern.
Jedoch hat Formaldehyd ernsthafte Sicherheitsbedenken hinsichtlich seiner Karzinogenität, allergischen Reaktionen und der Inhalationsrisiken sowohl in beruflichen als auch in Verbraucherszenarien aufgeworfen.
Um die Exposition gegenüber diesen potenziell krebserregenden Stoffen zu verringern, hat das Washington Department of Ecology am 28. August 2025 eine neue Regel angenommen, Kapitel 173-399 WAC: Kosmetikproduktbeschränkungen, die das Toxic-Free Cosmetics Act (TFCA) des Staates erweitert, um die Verwendung von Formaldehyd und allen formaldehydfreisetzenden Chemikalien in kosmetischen und Körperpflegeprodukten zu verbieten.
4. Geltungsbereich
Diese Regel gilt für alle kosmetischen Produkte, die zur Anwendung am oder im menschlichen Körper für Reinigung, Verschönerung, Förderung der Attraktivität oder Veränderung des Aussehens bestimmt sind. Sie umfasst:
Produkte, die in physischen Geschäften oder online im Bundesstaat Washington verkauft werden.
Kosmetika, die in professionellen Dienstleistungen verwendet werden (z. B. in Salons).
Sowohl fertige Produkte als auch solche, die zum Wiederverkauf verteilt werden.
Das Verbot untersagt speziell das absichtliche Hinzufügen von Formaldehyd oder den genannten Freisetzern - Spurenverunreinigungen aus anderen Quellen sind nicht eingeschränkt.
5. Verbotene Stoffe
Die Regel identifiziert 25 formaldehydfreisetzende Stoffe, die nicht absichtlich hinzugefügt werden dürfen. Diese werden häufig als Konservierungsmittel oder in Formulierungen wie Nagellacken und Haarprodukten verwendet. Die vollständige Liste umfasst:
| Artikel | Substanzname | CAS-Nummer |
| 1 | DMDM-Hydantoin | 6440-58-0 |
| 2 | Diazolidinyl-Urea | 78491-02-8 |
| 3 | Imidazolidinyl-Urea | 39236-46-9 |
| 4 | Quaternium-15 | 4080-31-3; 51229-78-8 |
| 5 | Tosylamid/Formaldehydharz (PTSAF) | 25035-71-6 |
| 6 | 2-Bromo-2-Nitropropan-1,3-Diol (Bronopol) | 52-51-7 |
| 7 | Natriumhydroxymethylglycinat | 70161-44-3 |
| 8 | Polyoxymethylen-Urea | 9011-05-6; 68611-64-3 |
| 9 | Polyoxymethylen-Melamin | 9003-08-1 |
| 10 | 5-Bromo-5-Nitro-1,3-Dioxan (Bronidox) | 30007-47-7 |
| 11 | 7-Ethylbicyclo-oxazolidin (Bioban CS1246) | 7747-35-5 |
| 12 | Benzylhemiformal/td> | 14548-60-8 |
| 13 | Dimethylhydantoin-Formaldehyd (DMHF) | 26811-08-5; 9065-13-8 |
| 14 | Dimethylol-Glykol | 3586-55-8 |
| 15 | Dimethylol-Urea | 140-95-4 |
| 16 | Dimethyloxazolidin | 51200-87-4 |
| 17 | MDM-Hydantoin | 116-25-6; 27636-82-4; 16228-00-5 |
| 18 | Methenamin | 100-97-0 |
| 19 | Methylal | 109-87-5 |
| 20 | Paraformaldehyd | 30525-89-4 |
| 21 | Polyoxymethylen | 9002-81-7 |
| 22 | Tetramethylolglykoluril | 5395-50-6 |
| 23 | Timonacic (bei Verwendung in wärmeaktivierten Haarglättern) | 444-27-9 |
| 24 | Tris-Hydroxymethylnitromethan | 126-11-4 |
| 25 | Urea, polymer with formaldehyde, isobutylated | 68002-18-6 |
6. Durchsetzung
| Anforderung | Datum des Inkrafttretens | |
| Verbot von Formaldehydfreisetzern | Keine neue Herstellung, Verkauf oder Vertrieb von Kosmetika mit absichtlich hinzugefügten Formaldehydfreisetzern | 1. Januar 2027 |
| Abverkauf von Lagerbeständen | Unternehmen ermöglichen, sich zu bereinigen, und Einzelhändlern, den Lagerbestand zu verwalten. Danach müssen die Produkte aus den Regalen verschwinden. | Erlaubt bis 31. Dezember 2027 |
Das Washington State Department of Ecology wird die Einhaltung überprüfen durch:
Überprüfung von Produktformulierungen.
Probenahme und Tests.
Andere relevante Datenquellen.
Kennzeichnung und Inhaltsangaben sollten mit den Bundesvorgaben übereinstimmen, es könnte jedoch eine staatsspezifische Überprüfung erforderlich sein.
In Kanada, wenn Gefahren in Konsumgütern identifiziert werden, werden sie zurückgerufen und in der Recalls and Safety Alerts Database auf der Website von Health Canada veröffentlicht, die täglich aktualisiert wird. Die Rückrufe in Kanada vom 01. August 2025 bis 31. August 2025 sind nachfolgend zusammengefasst:
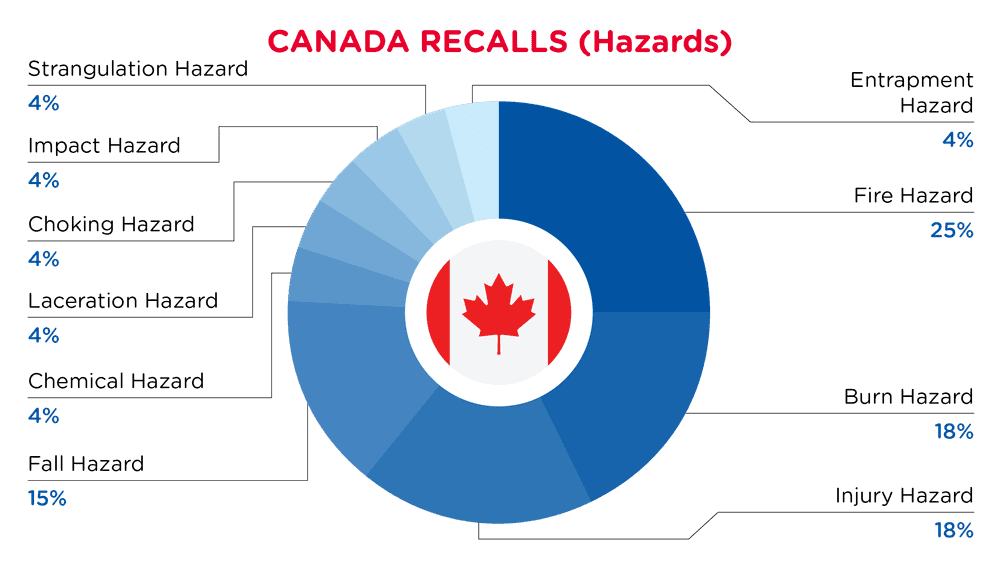
| Gefahren | Frequenz |
| Brandgefahr | 7 |
| Verbrennungsgefahr | 5 |
| Verletzungsgefahr | 5 |
| Sturzgefahr | 4 |
| Chemische Gefährdung | 1 |
| Gefahr von Schnittverletzungen | 1 |
| Erstickungsgefahr | 1 |
| Auswirkung Gefährdung | 1 |
| Gefahr der Strangulierung | 1 |
| Gefahr des Einklemmens | 1 |
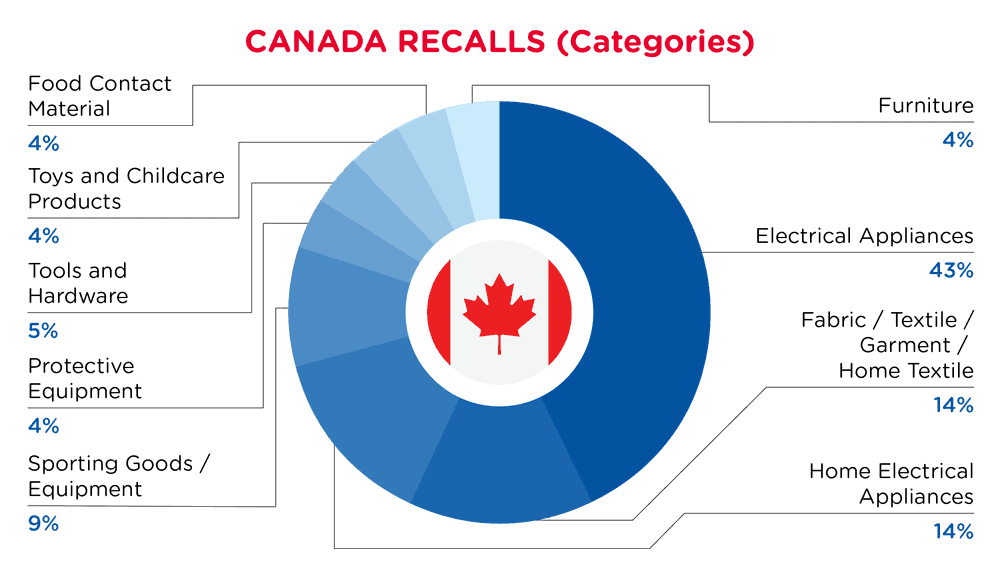
| Produkt-Kategorien | Frequenz |
| Elektrische Geräte | 9 |
| Stoff / Textil / Bekleidung / Heimtextilien | 3 |
| Elektrische Haushaltsgeräte | 3 |
| Sportartikel / Ausrüstung | 2 |
| Schutzausrüstung | 1 |
| Werkzeuge und Hardware | 1 |
| Spielzeug und Kinderpflegeprodukte | 1 |
| Material mit Lebensmittelkontakt | 1 |
| Möbel | 1 |
Für eine vollständige Liste klicken Sie hier
Neuigkeiten aus Europa
In Europa, wenn Gefahren in Nicht-Lebensmittel-Verbraucherprodukten identifiziert werden, werden die Produkte zurückgerufen und im Safety Gate-System veröffentlicht, das wöchentlich aktualisiert wird. Die europäischen Rückrufe vom 01. August 2025 bis zum 31. August 2025 sind unten zusammengefasst:
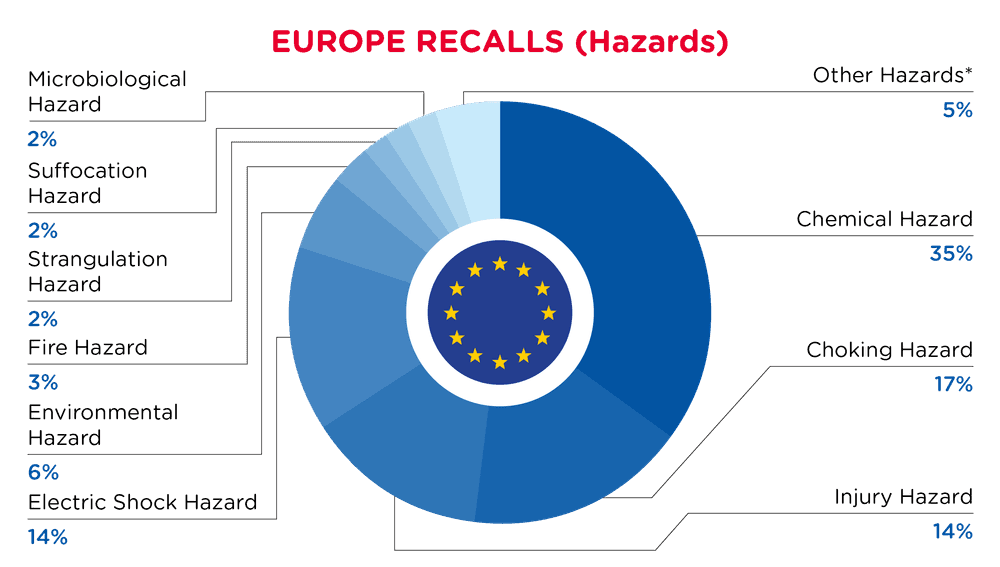
| Gefahren | Frequenz |
| Chemische Gefährdung | 108 |
| Erstickungsgefahr | 54 |
| Verletzungsgefahr | 43 |
| Gefahr eines elektrischen Schlages | 42 |
| Umweltgefährdung | 18 |
| Brandgefahr | 11 |
| Gefahr der Strangulierung | 8 |
| Erstickungsgefahr | 5 |
| Mikrobiologische Gefährdung | 5 |
| Andere Gefährdungen* | 15 |
*Andere Gefahren umfassen Hörschäden, Brandgefahr, Sehschäden, Einklemmen-Gefahr und Gesundheitsrisiko mit einer Häufigkeit von weniger als 5.
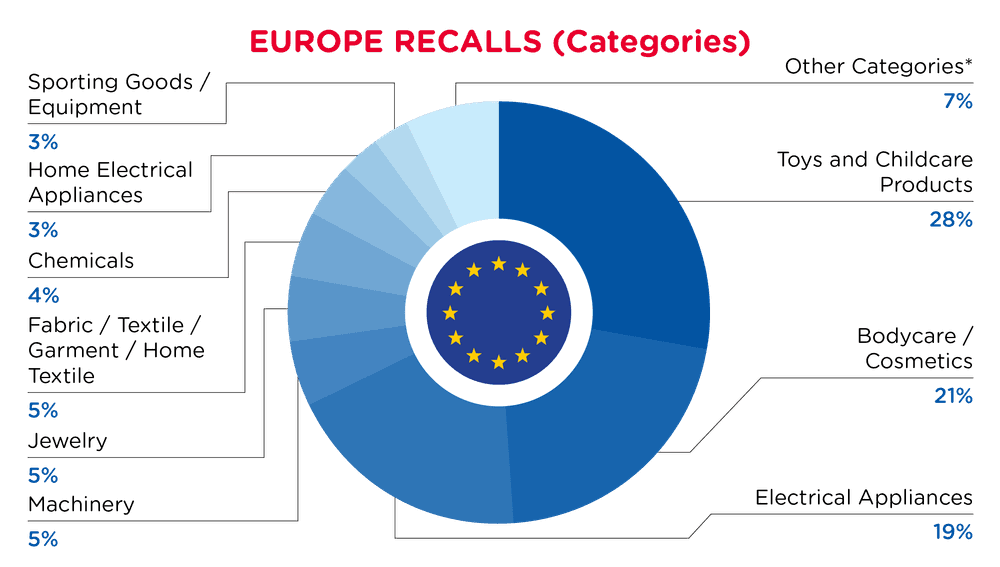
| Produkt-Kategorien | Frequenz |
| Spielzeug und Kinderpflegeprodukte | 69 |
| Körperpflege / Kosmetika | 52 |
| Elektrische Geräte | 46 |
| Maschinenpark | 13 |
| Schmuck | 12 |
| Stoff / Textil / Bekleidung / Heimtextilien | 12 |
| Chemikalien | 10 |
| Elektrische Haushaltsgeräte | 8 |
| Sportartikel / Ausrüstung | 7 |
| Andere Kategorien * | 17 |
*Andere Kategorien umfassen Schreibwaren, Computer / Audio / Video / Andere Elektronik & Zubehör, Outdoor-Living-Produkte, Schutzkleidung, Schuhe, Tierbedarf, Möbel und Zubehör mit einer Häufigkeit von weniger als 7.

| Notifizierendes Land | Frequenz |
| Ungarn | 47 |
| Schweden | 32 |
| Frankreich | 28 |
| Tschechische Republik | 23 |
| Deutschland | 19 |
| Polen | 18 |
| Vereinigtes Königreich im Hinblick auf Nordirland | 17 |
| Litauen | 11 |
| Slowakei | 10 |
| Italien | 8 |
| Österreich | 8 |
| Andere Länder* | 25 |
*Andere Länder umfassen Irland, Norwegen, Zypern, Spanien, Kroatien, Finnland, die Niederlande, Malta und Belgien mit einer Häufigkeit von weniger als 8.
Für die vollständige Liste klicken Sie hier
Die Europäische Kommission hat am 10. September 2025 einen Entwurf zur Änderung herausgegeben, um die Terminologie, den Geltungsbereich und die Fristen für die Verwendung von BPA in der Verordnung (EU) 2024/3190 zu korrigieren.
Am 10. September 2025 hat die Europäische (EU) Kommission einen Verordnungsentwurf vorgeschlagen, um die Kommissionsverordnung (EU) 2024/3190 zur Verwendung von Bisphenol A (BPA) und anderen Bisphenolen und Bisphenol-Derivaten zu überarbeiten. Diese Überarbeitung soll bestimmte Unklarheiten und Fehler klären, die wichtig sind, um die ordnungsgemäße Durchführung der Verordnung sicherzustellen.
Die folgende Tabelle kann hilfreich sein, um die Überarbeitungen schnell zu überprüfen:
Tabelle 1 - Überarbeitungen der Kommissionsverordnung (EU) 2024/3190.
| Artikel der (EU) 2024/3190 | Vergleich der Überarbeitungen im Absatz | Anmerkung |
| Artikel 3 Verbot der Verwendung von BPA | 1. Die Verwendung von BPA in der Herstellung von Materialien und Gegenständen, die mit Lebensmitteln in Berührung kommen, ist verboten. 2. Abweichend von Absatz 1 kann BPA in der Herstellung von Materialien, die mit Lebensmitteln in Berührung kommen, verwendet werden…… | In Artikel 3 der Verordnung (EU) 2024/3190 ist der Verweis auf ‚BPA und seine Salze‘ inkonsistent mit der Definition von ‚Bisphenol‘, die in Artikel 2(2)(c) festgelegt ist. Daher sollten die Worte ‚und seine Salze‘ aus Artikel 3 gestrichen werden. from Article 3. |
| Artikel 9 Überprüfung der Einhaltung der Anforderungen dieser Verordnung | 2. Zur Auswahl der Methoden, die verwenden werden, um zu überprüfen, dass ein Material oder Gegenstand, das/die nicht BPA-Rückstände, ein anderes gefährliches Bisphenol oder ein gefährliches Bisphenol-Derivat enthält…… (c) um zu überprüfen, dass ein Material oder Gegenstand, das/die nicht BPA-Rückstände, ein anderes gefährliches Bisphenol oder ein gefährliches Bisphenol-Derivat enthält, soll eine Extraktionsmethode verwendet werden. | Artikel 9(2) der Verordnung (EU) 2024/3190 zielt darauf ab, sicherzustellen, dass eine geeignete Analysemethode verwendet wird, um die Einhaltung von Artikel 4 dieser Verordnung zu überprüfen. Da Artikel 4 das Vorhandensein von ‚BPA-Rückständen‘ verbietet, sollte Artikel 9(2)(c) dieser Verordnung ebenfalls auf ‚BPA-Rückstände‘ verweisen. |
| Artikel 11 Übergangsbestimmungen zu Einweg-Lebensmittelkontaktartikeln | 1. Einweg-Lebensmittelkontaktartikel, die unter Verwendung von BPA hergestellt wurden und den vor dem Inkrafttreten dieser Verordnung geltenden Regeln entsprechen, die jedoch nicht den in dieser Verordnung festgelegten Regeln entsprechen, dürfen erstmals bis zum 20. Juli 2026 in Verkehr gebracht werden. 2. Abweichend von Absatz 1 können Materialien und Gegenstände, die nicht den in dieser Verordnung festgelegten Regeln entsprechen, erstmals bis zum 20. Januar 2028 in Verkehr gebracht werden. | Artikel 11 dieser Verordnung zielt darauf ab, Übergangsbestimmungen für die erstmalige Inverkehrbringung von Einweg-Lebensmittelkontaktartikeln bereitzustellen. Da Artikel 11 jedoch irrtümlich nur auf Materialien und Gegenstände, die ‚in Verkehr gebracht‘ werden, verweist, sollte er korrigiert werden. |
| Artikel 12 Übergangsbestimmungen hinsichtlich mehrfach verwendbarer End-Lebensmittelkontaktartikel | 3. Mehrfach verwendbare End-Lebensmittelkontaktartikel, die erstmals in Übereinstimmung mit Absatz 1 in Verkehr gebracht wurden (dürfen bis zum) 20. Juli 2027 auf dem Markt bleiben. Mehrfach verwendbare End-Lebensmittelkontaktartikel, die erstmals gemäß Absatz 2 in Verkehr gebracht wurden, dürfen bis zum 20. Januar 2029 auf dem Markt bleiben. | Das Ende des Übergangszeitraums für Lebensmittelkontaktartikel in Absatz 1 unterscheidet sich vom Ende des Übergangszeitraums in Absatz 2. Das angegebene Datum des 20. Januar 2029 gilt nur für Materialien und Gegenstände, die erstmals in Übereinstimmung mit Absatz 2 in Verkehr gebracht wurden. |
| ANLAGE III Die in Artikel 8 genannte Konformitätserklärung muss folgende Angaben enthalten | (3) die Identität von Zwischenprodukten der Lebensmittelkontaktartikel fertig. | Das Ziel ist es, die Identität von Materialien und Gegenständen, die mit Lebensmitteln in Berührung kommen, von einem Geschäft zu einem anderen zu verlangen. Daher sollte die Formulierung korrigiert werden. |
Diese Änderung tritt am zwanzigsten Tag nach ihrer Veröffentlichung im Amtsblatt der Europäischen Union in Kraft.
Hersteller sollten auf die Herstellung und Lagerung achten, um sicherzustellen, dass sie die aktualisierten Anforderungen erfüllen.
Die ECHA hat eine Konsultationsphase für die mögliche Aufnahme von drei Chemikalien als SVHC angekündigt. Sollten diese Einträge genehmigt werden, wird die Anzahl der als SVHC anerkannten Substanzen auf der Kandidatenliste möglicherweise auf 254 Einträge aktualisiert.
Am 1. September 2025 startete die Europäische Chemikalienagentur (ECHA) eine 45-tägige öffentliche Konsultation zu drei neuen potenziellen Stoffen von sehr hoher Besorgnis (SVHC), wie in der untenstehenden Tabelle gezeigt.
Am 27. Juni 2025 startete die Europäische Chemikalienagentur (ECHA) eine 45-tägige öffentliche Konsultation zu dem Stoff Decabromdiphenylethan (DBDPE) als neuer potenzieller SVHC.
Wenn die drei neuesten Substanzen genehmigt werden [zusammen mit DBDPE (CAS-Nummer: 84852-53-9)], wird die Anzahl der SVHCs auf der Kandidatenliste von 250 Einträgen auf 254 Einträge aktualisiert.
Es wird entschieden, ob diese Substanzen nach Abschluss der Konsultationsphase in die ECHA-Kandidatenliste der SVHC aufgenommen werden.
| Name des Stoffes | EG-Nummer | CAS-Nummer | Grund für die Aufnahme | Mögliche Anwendung |
| 4,4'-[2,2,2-trifluoro-1-(trifluormethyl)ethylidene]diphenol und seine Salze | - | - | Reproduktionstoxisch (Artikel 57c) | Herstellung von Polymeren und Gummi (z. B. reaktive Prozessregulatoren, Vulkanisierungsagenten oder Vernetzer) |
| 4,4'-Methylendiphenol | 210-658-2 | 620-92-8 | Reproduktionstoxisch (Artikel 57c) | Ersatz für andere Bisphenole in Polymeren, Kunststoffen oder Thermopapier Lacke, Lackierungen, Beschichtungen, Klebstoffe, Wasserrohre und Zahnversiegelungen |
| n-Hexan | 203-777-6 | 110-54-3 | Spezifische Zielorgan-Toxizität nach wiederholter Exposition (Artikel 57(f) - menschliche Gesundheit) | Beschichtungen, Polituren und Wachsmischungen Parfüme, Duftstoffe Kosmetika, Körperpflegeprodukte Reinigungsmittel, Treibmittel, Funktionelle Fluide, Polymerverarbeitung |
Die Europäische Direktion für die Qualität von Arzneimitteln & Gesundheitswesen hat kürzlich Ausgabe herausgegeben.
Am 1. September 2025 hat die Europäische Qualitätsagentur für Arzneimittel & Gesundheitswesen (EDQM) einen neuen technischen Leitfaden veröffentlicht, der die Resolution CM/Res (2020)9 des Europarates zur Sicherheit und Qualität von Materialien und Artikeln für den Kontakt mit Lebensmitteln ergänzt und die Erklärung zur Politik des Europarates zu Korkstopfen und anderen Korkmaterialien und Artikeln, die mit Lebensmitteln in Berührung kommen sollen (Version 2 vom 05.09.2007) ersetzt.
Der neue technische Leitfaden der EDQM aktualisiert wichtige Anforderungen an die Übereinstimmung für Korkmaterialien im Lebensmittelkontakt. Die folgende Tabelle kann hilfreich sein, um die wesentlichen Änderungen schnell zu überblicken:
Tabelle 1 - Zusammenfassung des technischen Leitfadens der EDQM
| Artikel | Technischer Leitfaden der EDQM für Kork (Inhalt und wichtige Änderungen) | Anmerkung |
| Geltungsbereich und Definitionen | Gilt für Korkmaterialien und Artikel, die mit Lebensmitteln in Berührung kommen. Der Hauptbestandteil dieser Materialien und Artikel ist Kork, der aus der Korkeiche geerntet wird (ISO 633: 2019). | z.B. Korkstopfen, Tabletts, Platzsets und Obstschalen. |
| Spezifische Anforderungen | Kein obligatorischer Bedarf entweder mit der QMA* oder spezifischem Migrationslimit SML** für Pentachlorphenol (PCP) und Trichlorphenol (TriCP). * QMA ist die maximal zulässige Restmenge einer Substanz im endgültigen Material oder Artikel, ausgedrückt als Gewicht pro Fläche des mit Lebensmitteln in Kontakt kommenden Artikels ** SML ist das spezifische Migrationslimit | PCP und TriCP sind allgemeine Anforderungen in der vorherigen Erklärung zur Politik. |
| Autorisierte Substanzen | Die Liste wurde aktualisiert. | / |
| Konformitätsprüfung | Erfüllen Sie die für die in der autorisierten Liste genannten Substanzen geltenden Einschränkungen. Aktive Substanzen dürfen verwendet werden, wenn diese Produkte für ihre Verwendung zugelassen wurden. Nicht überschreiten der maximalen Rückstandsmengen von Pestiziden in Lebensmitteln gemäß der Verordnung (EG) Nr. 396/2005 (anwendbar auf Produkte pflanzlichen und tierischen Ursprungs oder deren Teile, die als frische, verarbeitete und/oder zusammengesetzte Lebensmittel oder Futtermittel verwendet werden sollen). Darf keine Pilze, Pilztoxine oder Bakterien enthalten. | / |
| Testbedingungen und Analysemethoden | Die in der Verordnung (EU) Nr. 10/2011 über Kunststoffmaterialien und Gegenstände, die dazu bestimmt sind, mit Lebensmitteln in Kontakt zu kommen, festgelegten Lebensmittelsimulanzien und Konformitätsprüfungsbedingungen, sowie der JRC-Leitfaden zu Testbedingungen für Küchengeräteartikel im Kontakt mit Lebensmitteln sind zu beachten. | / |
| Konformitätserklärung (DoC) | Der technische Leitfaden der EDQM unterstreicht die Bedeutung der DoC und listet die Informationen auf, die die DoC enthalten sollte. | / |
Dieser Leitfaden kann sofort angewendet werden.
Hersteller sollen die Korkformulierungen und Produktionsprozesse bewerten und der Konformitätserklärung besondere Beachtung schenken, um sicherzustellen, dass sie die aktualisierte autorisierte Substanzliste und technische Anforderungen erfüllen.
Der aktualisierte EU-Vorschlag zur Beschränkung von PFAS, veröffentlicht von der ECHA am 20. August 2025, zielt auf die Herstellung, das Inverkehrbringen und die Verwendung von über 10.000 PFAS-Substanzen gemäß der REACH-Verordnung ab. Der Fokus liegt auf 14 Schlüsselsektoren, darunter Konsumgüter wie Textilien, Verpackungen und Kosmetika.
Der Vorschlag weicht von einem pauschalen Verbot ab und führt flexible Beschränkungsoptionen und Emissionskontrollen ein, um den Umweltschutz mit der Industrieumsetzbarkeit in Einklang zu bringen. Diese Maßnahmen fördern die Handhabbarkeit, indem sie eine weitere Nutzung in kritischen Anwendungen erlauben. Der Vorschlag erfordert von den Konsumgüterindustrien, sich in Übergangsperioden durch Tests und die Entwicklung von Alternativen auf die Einhaltung vorzubereiten.
Stellungnahmen zum Vorschlag werden für 2026 erwartet.
Am 20. August 2025 veröffentlichte die Europäische Chemikalienagentur (ECHA) einen aktualisierten Vorschlag zur Beschränkung von per- und polyfluorierten Alkylsubstanzen (PFAS)—umgangssprachlich als "Forever Chemicals" bekannt—unter der Registrierung, Bewertung, Autorisierung und Beschränkung chemischer Stoffe (REACH). Diese Überarbeitung folgt auf eine umfassende Prüfung von über 5.600 Stakeholder-Kommentaren aus der ersten Konsultation 2023. Der aktualisierte Bericht, bekannt als Hintergrunddokument, dient als Grundlage für die Stellungnahmen der ECHA-Ausschüsse für Risikobeurteilung (RAC) und sozioökonomische Analyse (SEAC). Der Vorschlag, angeführt von Behörden aus Dänemark, Deutschland, den Niederlanden, Norwegen und Schweden, zielt darauf ab, PFAS-Emissionen in die Umwelt zu minimieren und die menschliche Gesundheit zu schützen, indem Risiken von mehr als 10.000 PFAS-Substanzen in Hauptsektoren adressiert werden.
Während der ursprüngliche Vorschlag ein breites "universelles" Verbot in Betracht zog, nähert sich die Aktualisierung mit einem raffinierteren Ansatz, indem spezifische Ausnahmen und gestaffelte Umsetzungstermine eingeführt werden, um ein Gleichgewicht zwischen Umweltzielen und praktischer Umsetzbarkeit zu finden. Dies ist besonders relevant für die Konsumgüterindustrie, wo PFAS in Artikeln wie Textilien, Verpackungen und Kosmetik verwendet werden.
1. Anwendungsbereich
Die Beschränkung zielt auf die Herstellung, das Inverkehrbringen und die Verwendung von PFAS in der EU ab, mit Schwerpunkt auf gezielten Produkt- und Prozesszusätzen. Sie gilt für eine Vielzahl von Sektoren, aber aufgrund von Bewertungsterminen deckt die anfängliche Beschränkung nur die ursprünglich im Dossier 2023 ermittelten 14 Sektoren ab. Acht weitere Sektoren, die durch Konsultationsfeedback identifiziert wurden, werden nicht in die Stellungnahmen des Ausschusses 2026 aufgenommen und erfordern möglicherweise separate zukünftige Bewertungen.
| Kategorie | Abgedeckte Sektoren (Ursprüngliche 14) | Ausgeschlossene Sektoren (8 Neue Sektoren – Verschoben) |
| Konsumentenfokussiert | - Textilien und Bekleidung - Papier und Verpackungen (z. B. lebensmittelkontaktmaterialien) - Kosmetika - Persönliche Schutzausrüstung (PSA) | - Druckanwendungen - Technische Textilien |
| Industriell und Technisch | - Beschichtungen und Lacke - Bauprodukte - Schmierstoffe - Elektronik und Halbleiter - Transport (z. B. Fahrzeuge) - Energie (z. B. Wärmeübertragungsflüssigkeiten, Batterien) | - Dichtungsanwendungen - Maschinenanwendungen - Umfangreichere industrielle Nutzung (z. B. Lösungsmittel, Katalysatoren) |
| Spezialisiert | - Medizinische Geräte - Fluorierte Gase und Kältemittel | - Andere medizinische Anwendungen (z. B. Arzneimittelverpackungen) - Militärische Anwendungen - Sprengstoffe |
2. Beschränkte Optionen (ROs)
Der ursprüngliche Vorschlag legte zwei Beschränkungsoptionen (RO) fest: RO1, ein vollständiges PFAS-Verbot mit einer Übergangsfrist von 18 Monaten, und RO2, ein Verbot mit sektorspezifischen Ausnahmen von 5 bis 12 Jahren, ebenfalls mit einer vorausgehenden Übergangsfrist von 18 Monaten. Als Reaktion auf Input von Interessengruppen prüften die Behörden zudem eine dritte Option (RO3), die bestimmte PFAS-Verwendungen unter strengen lebenszyklusbezogenen Emissionskontrollen zulassen würde.
Der aktualisierte Vorschlag bewertet diese Beschränkungsoptionen (ROs) unter Betonung risikobasierter Kontrollen. Der Kernansatz ist ein allgemeines Verbot von PFAS, mit maßgeschneiderten Ausnahmen, um eine weitere Nutzung zu erlauben, wenn Alternativen nicht verfügbar sind oder Risiken angemessen verwaltet werden können.
| Beschränkte Option | Beschreibung | Abdeckung/Beschränkungen |
| RO1: Vollständiges Verbot | Vollständiges Verbot von Herstellung, Inverkehrbringen und Nutzung von PFAS, ohne Ausnahmen. | Gilt allgemein, wird jedoch aufgrund von Umsetzbarkeitsbedenken nicht als primäre Option übernommen; dient als Vergleichsgrundlage. |
| RO2: Verbot mit spezifischen Ausnahmen | Allgemeines Verbot, jedoch wird die Nutzung von PFAS für 5–12 Jahre (plus 18-monatiger Übergangsfrist) in spezifischen Anwendungen erlaubt, wo noch keine tragfähigen Alternativen verfügbar sind. Ausnahmen sind sektorspezifisch und unterliegen der Überprüfung. | - Deckt alle 14 ursprünglichen Sektoren ab. - Beispiele: 5 Jahre für Kosmetika; bis zu 12 Jahre für Halbleiter und medizinische Geräte. - Neue Ausnahmen für Gebrauchtartikel, Ersatzteile, recyceltes Papier/Karton und Kunststoffe über Konzentrationsschwellen. |
| RO3: Bedingte Nutzung unter Einschränkungen | Erlaubt die Nutzung von PFAS unter strengen Emissionsgrenzen über den gesamten Lebenszyklus und wurde für einige Sektoren in Betracht gezogen. | - Fokussiert auf Hightech-Sektoren wie Elektronik, Energie und PFAS-Produktion. - Umfasst unbefristete Ausnahmen für Forschung & Entwicklung (F&E) und Zwischenprodukte in ausgenommenen Prozessen. |
Die Beschränkungsoptionen RO1, RO2 und RO3 werden als ausreichend durchsetzbar betrachtet. RO1 dürfte nicht praktikabel oder handhabbar sein, während RO2 und RO3 als machbar und praktisch in Bezug auf Umsetzung, Vollzug und Management angesehen werden. Ergänzende Maßnahmen wurden ebenfalls in Kombination mit RO2 in Betracht gezogen, um das Risikomanagement zu stärken.
| Umfang von PFAS | Anforderung | Bedingungen der Einschränkung |
| Substanzen alleinstehend | Verbotene | Die anfängliche 18-monatige Schonfrist richtet sich an Anwendungen, bei denen Alternativen kurzfristig machbar sind. Für Anwendungen mit hohem mittelfristigem Substitutionspotenzial: 6,5 Jahre nach Inkrafttreten (EIF) Für Anwendungen mit geringem mittelfristigem Substitutionspotenzial: 13,5 Jahre nach EIF Kunststoffartikel, die recyceltes Material enthalten, außer Lebensmittelkontaktmaterialien (FCM) und Spielzeug: 23,5 Jahre nach EIF Zeitlich unbegrenzte Ausnahmen für Papier- und Kartonartikel, die recyceltes Material enthalten, mit Ausnahme von FCM und Verpackungen |
| Bestandteile einer anderen Substanz In Mischungen In Artikeln | 25 ppb für einzelne PFAS (gezielte Analyse) 250 ppb für die Summe der PFAS (mit optionaler Vorläuferabbau) 50 ppm für den gesamten Fluorgehalt (einschließlich polymerer PFAS) |
Wenn validierte Methoden nicht verfügbar sind, wird eine Gesamtfluoranalyse verwendet, wobei der Nachweis, ob das Fluor von PFAS stammt oder nicht, von Herstellern oder Importeuren erbracht werden muss. Alle drei Schwellenwerte gelten einheitlich für jedes Produkt, wobei Überschreitungen nur erlaubt sind, wenn das Produkt für eine spezifische Ausnahme, die im Entwurf der Beschränkung festgelegt ist, in Frage kommt.
3. Nächste Schritte
Nach der Veröffentlichung des Hintergrunddokuments, veröffentlichte die ECHA am 27. August 2025 eine Mitteilung, die ein Update ihrer Bewertung des Vorschlags und die erwartete Zeitleiste klärt.
Der Vorschlag befindet sich nun zur Bewertung in den wissenschaftlichen Ausschüssen der ECHA, wobei die Stellungnahmen für 2026 erwartet werden. RAC und SEAC planen, ihre Diskussionen über die 14 im ursprünglichen Beschränkungsvorschlag abgedeckten Sektoren plus PFAS-Produktion und horizontale Fragen bis Ende 2025 abzuschließen. ECHA wird die acht zusätzlichen im aktualisierten Vorschlag enthaltenen Sektoren nicht bewerten. Dies soll es der ECHA ermöglichen, die Stellungnahme des RAC und den Entwurf der SEAC-Stellungnahme abzuschließen und die Konsultation zum SEAC-Entwurf in der ersten Hälfte 2026 durchzuführen.
(Bemerkung: gemäß dem aktualisierten Beitrag der ECHA vom 15. September 2025 beabsichtigt SEAC, seinen Entwurf in seiner Sitzung zu vereinbaren, die vorläufig für die erste Hälfte März 2026 geplant ist.)
Die endgültigen Stellungnahmen von RAC und SEAC, zusammen mit dem Hintergrunddokument, sollen der Europäischen Kommission ermöglichen, fundierte Entscheidungen darüber zu treffen, wie die verschiedenen Sektoren (14 identifizierte Sektoren plus acht zusätzliche), die PFAS-Produktion und übergeordnete sektorübergreifende Fragen am besten angegangen werden können.
Die Europäische Kommission hat sich, in ihrem Aktionsplan für die Chemieindustrie vom 8. Juli 2025, verpflichtet, einen Legislativvorschlag "so bald wie möglich" nach Erhalt der ECHA-Stellungnahmen vorzulegen.
Frankreich hat eine Mitteilung veröffentlicht, um die Substanzen der „Liste der zugelassenen Stoffe in Anhang VIII“, die vor dem 1. Juli 2025 gemäß dem Dekret vom 5. August 2020 über Gummimaterialien, die in Materialien in Kontakt mit Lebensmitteln und Schnullern verwendet werden, einzureichen sind, zu reduzieren.
Am 11. August 2020 veröffentlichte die französische Regierung offiziell das Dekret vom 5. August 2020, das Gummimaterialien und -artikel regelt, die für den Kontakt mit Lebensmitteln und Schnuller für Kleinkinder bestimmt sind. Das Dekret vom 5. August 2020 ersetzt und hebt das vorherige Dekret vom 9. November 1994 auf.
Neben den Artikeln gibt es acht Anhänge zum Dekret vom 5. August 2020:
Anhang I Zugelassene Monomere, Ausgangsstoffe und Modifizierungsmittel
Anhang II Zugelassene Zusatzstoffe
Anhang III Prüfbedingungen für die Messung der spezifischen Migration und Gesamtmigration
Anhang IV N-Nitrosamine
Anhang V Konformitätserklärung
Anhang VI Freie flüchtige organische Verbindungen
Anhang VII Reinheitskriterien für Farbstoffe und Pigmente
Anhang VIII Liste der zugelassenen Stoffe, die der Einreichung des notwendigen Dossiers zur Bewertung vor dem 1. Juli 2025 unterliegen
In dem obigen Anhang VIII sind bereits sechzig Stoffe aufgeführt.
Am 7. August 2025 veröffentlichte die französische Regierung die Mitteilung, um die zugelassenen Stoffe im obigen Anhang VIII von 60 auf 10 zu reduzieren. Die Stoffe in Anhang VIII, die nicht in der Mitteilung enthalten sind, dürfen nicht mehr verwendet werden, es sei denn, sie erfüllen die Anforderungen von Artikel 10 des Dekrets vom 5. August 2020, wonach Stoffe von der Europäischen Behörde für Lebensmittelsicherheit oder einer kompetenten wissenschaftlichen Stelle in der Europäischen Union (EU), der Türkei oder dem Europäischen Wirtschaftsraum (EWR) bewertet oder anerkannt wurden.
Diese Mitteilung tritt sofort in Kraft. Es ist für relevante Unternehmen imperative, über die aktualisierten regulatorischen Anforderungen in ihren Produktionsprozessen informiert zu sein, um die Compliance zu erreichen.
Neuigkeiten aus China
In China werden bei der Identifizierung von Gefahren in Konsumgütern diese zurückgerufen und im SAMR Defective Product Administrative Centre, das täglich aktualisiert wird, veröffentlicht. Die Rückrufe in China vom 01. August 2025 bis 31. August 2025 werden unten zusammengefasst:
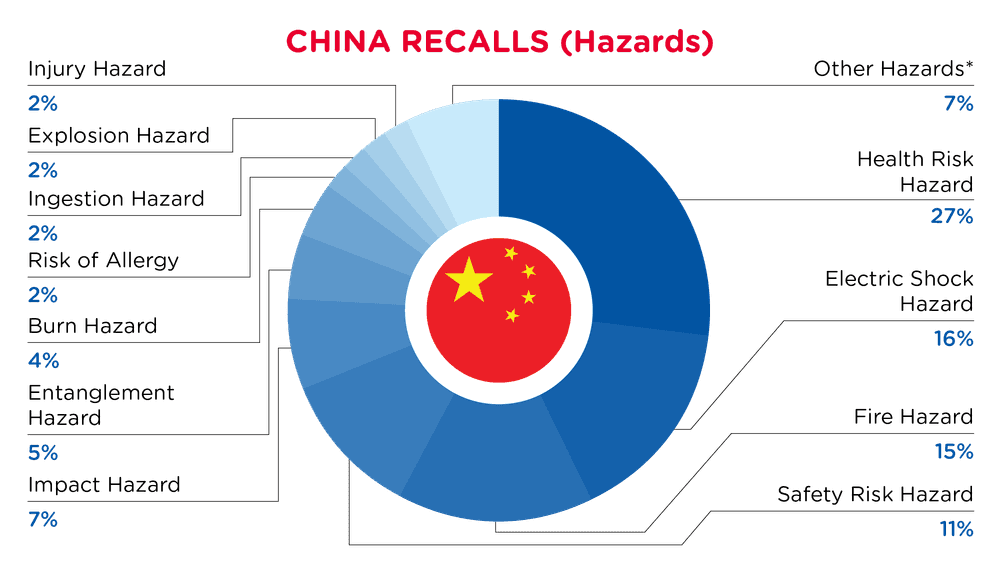
| Gefahren | Frequenz |
| Gesundheitsrisiko Gefährdung | 22 |
| Gefahr eines elektrischen Schlages | 13 |
| Brandgefahr | 12 |
| Sicherheitsrisiko Gefährdung | 9 |
| Auswirkung Gefährdung | 6 |
| Verhedderungsgefahr | 4 |
| Verbrennungsgefahr | 3 |
| Risiko einer Allergie | 2 |
| Gefahr des Verschluckens | 2 |
| Explosionsgefahr | 2 |
| Verletzungsgefahr | 2 |
| Andere Gefährdungen* | 6 |
*Andere Gefahren umfassen Unfall-, Sehkraftschäden-, Umkipp-, Schnitt-, Stich- und Erstickungsgefahren mit einer Häufigkeit von weniger als 2.
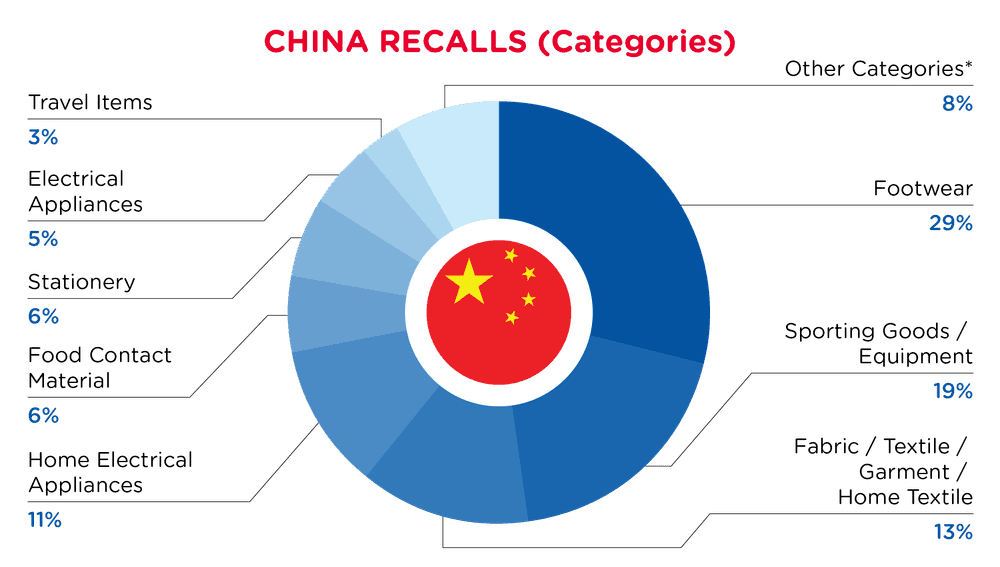
| Produkt-Kategorien | Frequenz |
| Schuhe | 18 |
| Sportartikel / Ausrüstung | 12 |
| Stoff / Textil / Bekleidung / Heimtextilien | 8 |
| Elektrische Haushaltsgeräte | 7 |
| Material mit Lebensmittelkontakt | 4 |
| Schreibwaren | 4 |
| Elektrische Geräte | 3 |
| Reiseartikel | 2 |
| Andere Kategorien* | 5 |
*Andere Kategorien umfassen Spielzeug und Kinderprodukte, Schmuck, Chemikalien, Möbel und Schutzausrüstungen mit einer Häufigkeit von weniger als 2.
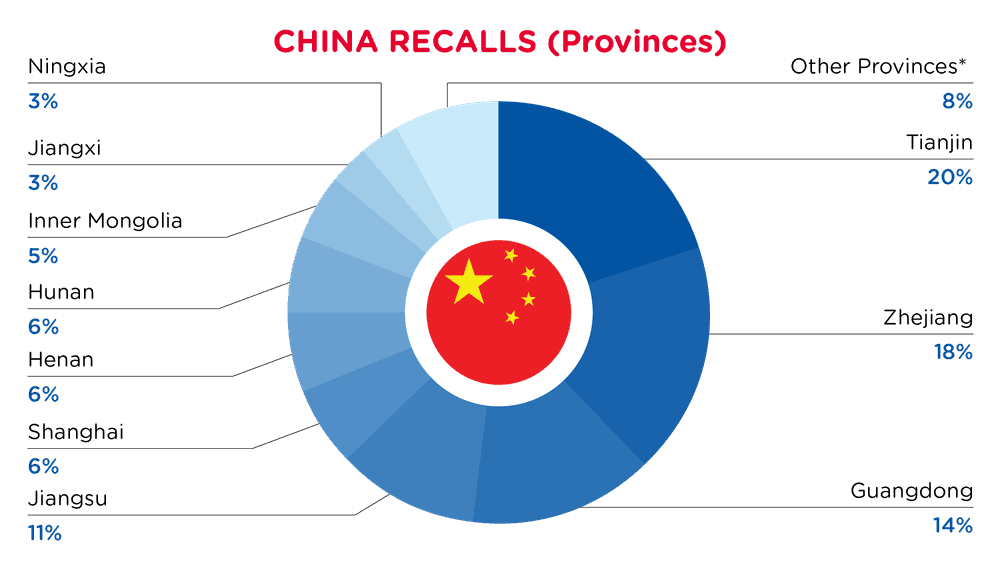
| Provinzen | Frequenz |
| Tianjin | 12 |
| Zhejiang | 11 |
| Guangdong | 9 |
| Jiangsu | 7 |
| Shanghai | 4 |
| Henan | 4 |
| Hunan | 4 |
| Innere Mongolei | 3 |
| Jiangxi | 2 |
| Ningxia | 2 |
| Andere Provinzen* | 5 |
*Andere Provinzen umfassen Chongqing, Gansu, Peking, Sichuan und Shanxi mit einer Häufigkeit von weniger als 2.
Für eine vollständige Liste klicken Sie hier.
Am 1. August 2025 veröffentlichte China offiziell GB 26572-2025, "Beschränkungen zur Verwendung gefährlicher Substanzen in elektrischen und elektronischen Geräten". Es ist Chinas erster obligatorischer RoHS-Standard und wird am 1. August 2027 in Kraft treten und den vorherigen GB/T 26572-2011-Standard vollständig ersetzen. Der neue Standard zielt darauf ab, die Umweltverschmutzung und Schäden für den Menschen zu reduzieren und die hochwertige Entwicklung der Branche in "grünere" und gesündere Richtungen zu fördern. Der Standard gilt für elektrische und elektronische Produkte, die in China hergestellt, verkauft und importiert werden.
GB 26572-2025 "Beschränkungen zur Verwendung gefährlicher Substanzen in elektrischen und elektronischen Geräten" ist Chinas erster obligatorischer nationaler Standard im Bereich der Beschränkung gefährlicher Substanzen (RoHS). Er ersetzt den vorherigen empfohlenen Standard GB/T 26572-2011, "Grenzwerte für beschränkte Substanzen in elektrischen und elektronischen Produkten", vollständig und markiert, dass Chinas Kontrolle über gefährliche Substanzen in elektrischen und elektronischen Produkten in die obligatorische Phase übergeht. Der Standard soll die hochwertige Entwicklung der Branche in eine "grünere" und gesündere Richtung lenken.
Der Standard wurde am 1. August 2025 von der Standardisierungsverwaltung Chinas genehmigt und veröffentlicht und wird am 1. August 2027 in Kraft treten. Nach der Umsetzung ist eine 13-monatige Übergangsfrist vorgesehen (bis zum 1. September 2028), um den Unternehmen die Möglichkeit zu geben, vorhandene Bestände abzubauen.
1. Anwendungsbereich
Dieses Dokument legt die Grenzwerte für gefährliche Substanzen in elektrischen und elektronischen Produkten und die Anforderungen an die Kennzeichnung fest.
Dieses Dokument gilt für elektrische und elektronische Produkte, die innerhalb des Territoriums der Volksrepublik China hergestellt, verkauft und importiert werden.
2. Wesentliche Änderungen des Standards
2.1 Erweiterung der kontrollierten Substanzen
Vier zusätzliche Phthalate wurden hinzugefügt (DBP, DIBP, BBP, DEHP). Die Gesamtzahl der kontrollierten Substanzen steigt von 6 auf 10, wobei die Grenzwerte vollständig mit EU RoHS übereinstimmen. Die vollständige Liste ist:
| Artikel | Substanzname | CAS-Nummer | Grenz % |
| 1 | Blei (Pb) | 7439-92-1 | ≤0,1 |
| 2 | Quecksilber (Hg) | 7439-97-6 | ≤0,1 |
| 3 | Cadmium (Cd) | 7440-43-9 | ≤0,01 |
| 4 | Chrom(VI) | 18540-29-9 | ≤0,1 |
| 5 | Polybromierte Biphenyle (PBBs) | --- | ≤0,1 |
| 6 | Polybromierte Diphenylether (PBDEs) | --- | ≤0,1 |
| 7 | Dibutylphthalat (DBP) | 84-74-2 | ≤0,1 |
| 8 | Diisobutylphthalat (DIBP) | 84-69-5 | ≤0,1 |
| 9 | Benzylbutylphthalat (BBP) | 85-68-7 | ≤0,1 |
| 10 | Bis(2‑ethylhexyl)phthalat (DEHP) | 117-81-7 | ≤0,1 |
2.2 Verfeinerte Klassifikationsverwaltung
Kategorie I Produkte (z.B. Kühlschränke, Mobiltelefone und andere 12 aufgelistete Produkttypen): müssen sowohl Konzentrationsgrenzen als auch Kennzeichnungsanforderungen erfüllen.
Kategorie II Produkte (andere elektrische und elektronische Produkte): Die Einhaltung der Konzentrationsgrenzen wird empfohlen; nur die Kennzeichnungsanforderungen sind verbindlich.
2.3 Aktualisierungen bei Prüf- und Kennzeichnungsverfahren
Die GB/T 39560-Serie (äquivalent zu IEC 62321 zur chemischen Analyse von Elektronik) wird einheitlich als Prüfverfahren übernommen, um die Konsistenz der Ergebnisse zu verbessern.
Digitale Kennzeichnungsoptionen wie QR-Codes und elektronische Displays werden hinzugefügt, um die Compliance-Kosten für Unternehmen zu senken.
2.4 Aktualisierungen der Terminologie
Neue Begriffe wurden hinzugefügt, z.B. "Umweltschutz-Lebensdauer" und "Katalog von Produkten, die die Anforderungen an die Einschränkung von gefährlichen Substanzen erfüllen", um die ökologische Verantwortung der Produktlebensdauer zu klären.
3. Durchsetzung
| Anforderung | Datum | |
| Beschränkungen zur Verwendung gefährlicher Substanzen in elektrischen und elektronischen Geräten | Produkte, die nach dem Inkrafttreten des Standards hergestellt werden, müssen die Prüfanforderungen des Standards erfüllen. | 1. August 2027 |
| Abverkauf von Lagerbeständen | Für Produkte, die vor der Umsetzung des Standards hergestellt oder importiert wurden, treten die Anforderungen dieses Dokuments ab dem 13. Monat nach der Umsetzung in Kraft. | Erlaubt bis 1. September 2028 |
Neuigkeiten aus Australien/Neuseeland
In Australien werden Verbraucherprodukte, bei denen Gefahren festgestellt werden, zurückgerufen und in der Datenbank für Rückrufe und Sicherheitswarnungen auf der Website der Australischen Wettbewerbs- und Verbraucherkommission veröffentlicht, die täglich aktualisiert wird. Die Rückrufe in Australien vom 01. August 2025 bis 31. August 2025 sind unten zusammengefasst:
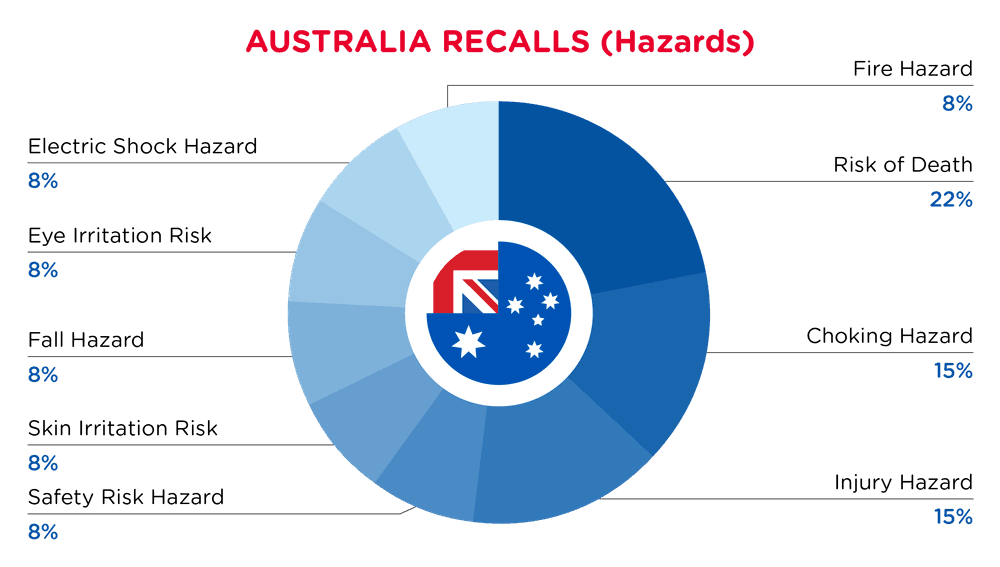
| Gefahren | Frequenz |
| Risiko des Todes | 3 |
| Erstickungsgefahr | 2 |
| Verletzungsgefahr | 2 |
| Sicherheitsrisiko Gefährdung | 1 |
| Risiko der Hautreizung | 1 |
| Sturzgefahr | 1 |
| Risiko der Augenreizung | 1 |
| Gefahr eines elektrischen Schlages | 1 |
| Brandgefahr | 1 |
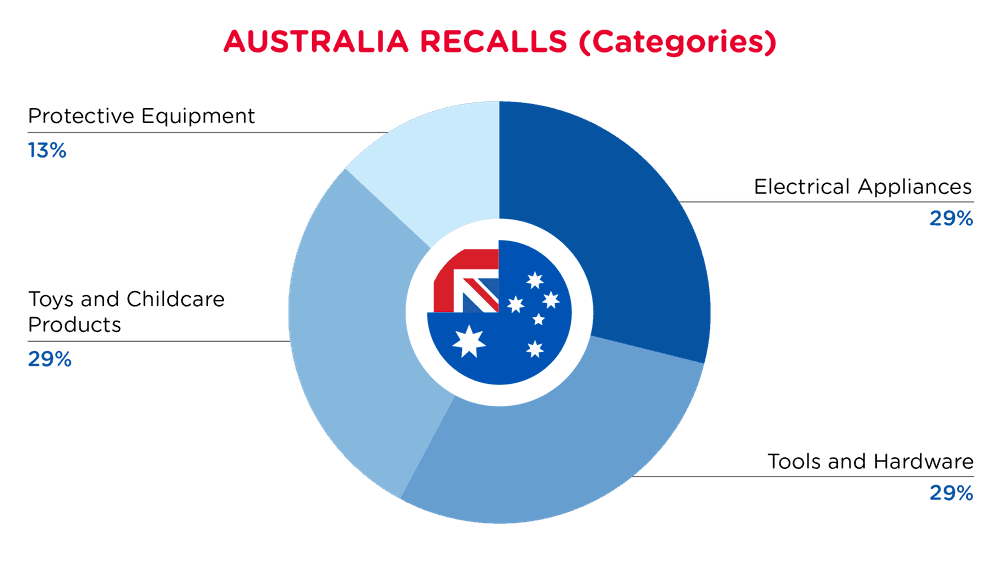
| Produkt-Kategorien | Frequenz |
| Elektrische Geräte | 2 |
| Werkzeuge und Hardware | 2 |
| Spielzeug und Kinderpflegeprodukte | 2 |
| Schutzausrüstung | 1 |
Für eine vollständige Liste klicken Sie hier
Abonnieren Sie unsere regulatorischen Updates
Sie können sich jederzeit abmelden. Lesen Sie unsere Datenschutzrichtlinie.